Research interests
Being able to find our way around familiar environments is essential in our daily lives, and impairments in this function, observed in several psychiatric and neurological disorders, are particularly debilitating. It is believed that effective navigation relies on learning through exploration of an internal representation of our environment, also known as a cognitive map. The hippocampus plays a central role in this process and also contributes to the memorization of past events in our daily lives (episodic memory).
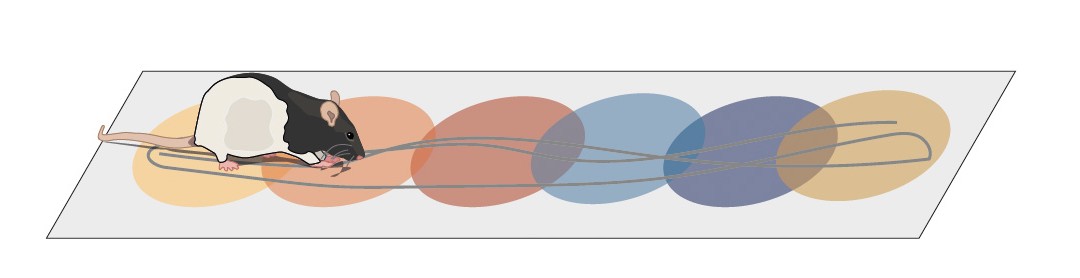
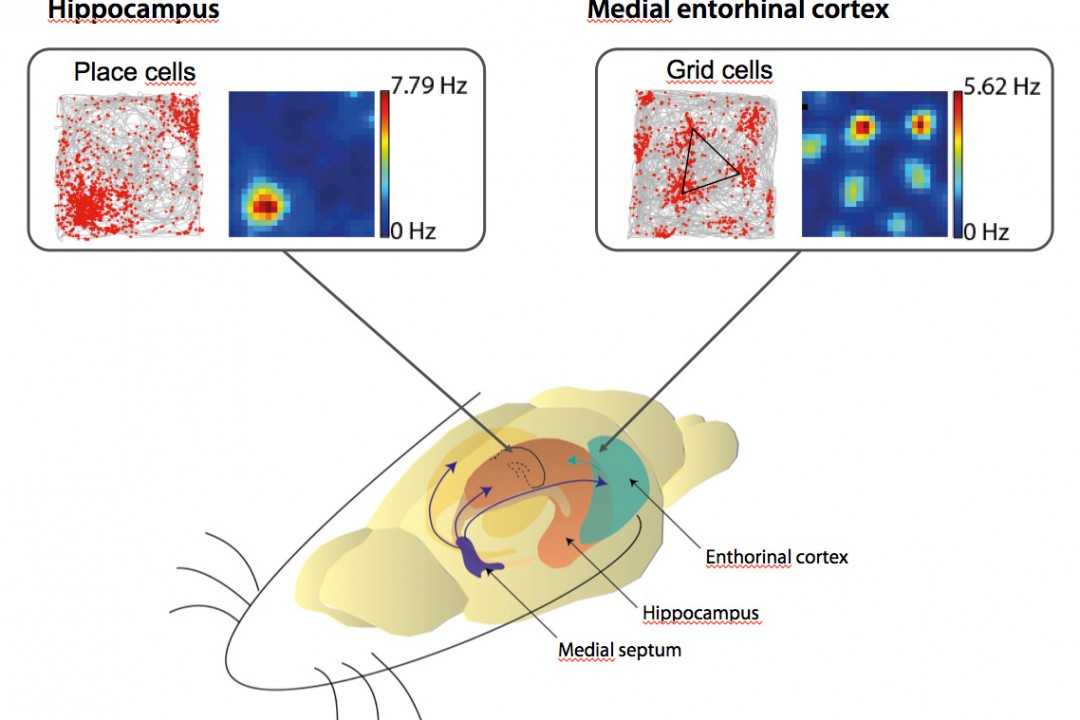
The activity of neurons in the hippocampus, called “place cells,” is spatially modulated: in environments rich in external landmarks, place cells increase their activity in specific locations called “place fields” relative to these cues. This is referred to as position coding. However, when external landmarks are not available, place cells can rely on sensory information related to movement (such as proprioception and vestibular information) to modulate their activity according to the distance traveled in specific directions, enabling navigation based on path integration. This is referred to as distance coding.
Unlike position coding, distance coding in the hippocampus is poorly understood and has been little studied. Our team was the first in France to develop the use of virtual reality for studying spatial cognition in rodents. We have shown that external visual cues influence the resolution of spatial information coding (Bourboulou, Marti et al., 2019). Recently, we were able to isolate hippocampal distance coding in the absence of local visual cues and demonstrate a specific contribution of grid cells in the entorhinal cortex to this coding (Norlund et al., 2025).
At the cellular level, our team participated in the development of innovative techniques for recording the membrane potential of hippocampal cells during exploration of real and virtual environments (Lee, Epsztein and Brecht 2009; Epsztein, Lee et al., 2010; ). These approaches have revealed a link between intrinsic cellular excitability and spatial information coding (Morgan et al., 2019; Epsztein et al., 2011).
Our current projects focus on the study of hippocampal coding during development (Valeeva et al., 2018) and in animal models of neurodevelopmental disorders associated with epileptic encephalopathies (Milh et al., 2020; Biba-Maazou et al., 2022) and autism spectrum disorders, and the cellular mechanisms involved.
Specific projects
- aRelative influence of internal and external sensory cues on spatial coding resolution and memory
- bGrid cells contribution to distance coding and update of cognitive maps
- cIntracellular determinants of place cells activation in new and familiar environments
Relative influence of internal and external sensory cues on spatial coding resolution and memory
PI: Jérôme Epsztein & Julie Koenig with Romain Bourboulou and Geoffrey Marti in collaboration with the team of Hervé Rouault
Every location in an environment is represented by the selective firing of an ensemble of place cells active there, among a larger population of silent neurons. This representation can be more or less precise depending on the size of this ensemble (with a higher proportion of place cells associated with a higher spatial coding resolution akin the number of pixels on a picture), the size of their place field (akin the size of the pixels) but also their spatial and temporal stability. The factors controlling spatial coding resolution are poorly understood. In this project we address this question taking advantage of recently developed virtual reality systems for rodents combined with high-density extracellular recordings (silicon probes). Virtual reality allows us a better control of the sensory cues that are available to animals for self-location. Our recent results (Bourboulou, Marti et al., 2019) suggest that spatial coding resolution could be modulated locally within the same environment. This could be relevant for navigating large scale/complex environments for rodents or memory space in humans.
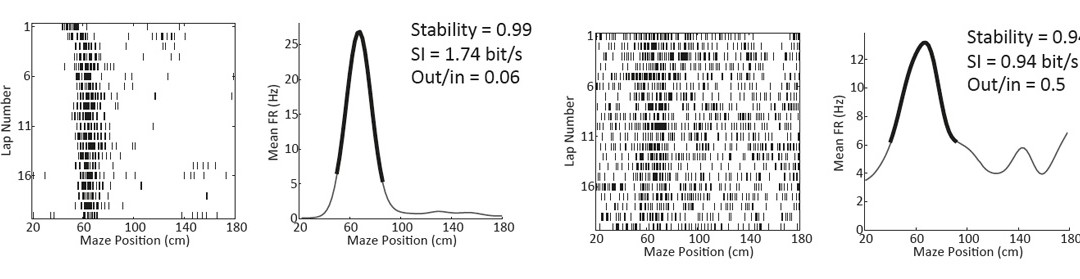
Grid cells contribution to distance coding and update of cognitive maps
P.I.: Julie Koenig with Mathilde Nordlund
Navigation using an internal representation of the environment or a cognitive map requires allothetic information about the spatial arrangement of the landmarks and idiothetic (self-motion) information. Grid cells in the medial entorhinal cortex (MEC) exhibit a striking grid-like firing pattern that tesselate the environment and are supposed to be mainly driven by idiothetic cues in order to compute distances travelled in the environment. However, this point of view has been challenged by several studies showing that grid cells are highly sensitive to external visual cues suggesting that these cues might be important in the establishment of grid cells firing patterns. Finally, the grid cells population might be functionally more heterogeneous than previously thought as they are present in two populations of projecting cells in layer 2 of the MEC that are characterized by different morpho-functional properties: stellate and pyramidal cells.
Our general goal is to test how grid cells encode distance travelled and how they react to local change in the availability of environmental landmarks. To do so we take advantage of the recent development of virtual reality systems for rodents, an efficient tool to modify environments instantaneously and in a very controlled and reliable way.
Intracellular determinants of place cells activation in new and familiar environments
P.I.: Jerome Epsztein with Peter Morgan
At the cellular level, despite decades of study, the intracellular mechanisms responsible for the recruitment of a given cell into the assembly coding an environment are still poorly understood. Why are some cells active rather than silent in a given environment? Are these cells selected randomly or following specific rules? Slice experiments have shown that the input/output transformation in CA1 pyramidal cells is highly non-linear because CA1 pyramidal cells dendrites are endowed with voltage-gated conductances that can generate regenerative local or global dendritic spikes. Our previous intracellular recordings of CA1 pyramidal cells from freely moving rats indicated the presence of plateau potentials (a signature of dendritic regenerative events) and burst firing (a signature of increased intrinsic excitability) specifically in spatially coding cells (Epsztein et al., 2011). Based on the above results we propose that the inital level of intrinsic excitability of CA1 pyramidal cells is central to the spatial modulation of their firing rate and their recruitment into the cell assembly coding a new environment. We currently investigate the role of long-term plasticity of intrinsic excitability (Morgan et al., 2019) in the regulation of memory allocation.

Collaborations
INMED:
External:
Dr. Judith Makara, Institute of Experimental Medicine of the Hungarian Academy of Sciences, Budapest, Hungary
Dr. Alex Roxin, Centre de Recerca Matemàtica, Barceona, Spain
Alumni
Romain Bourboulou (PhD 2015-2019, now post-doc in the Barry lab, UCL, London)
Caroline Filippi (Engineer 2015-2019)
Geoffrey Marti (Post-doc 2015-2019)
François-Xavier Michon (PhD 2014-2018, now post-doc in the Lacaille lab, Montreal)
Peter Morgan (Post-doc 2014-2019)
David Ouedraogo (PhD 2009-2013, now working at Johnston & Johnston)
Rachel Carayon (Engineer 2025)
Salomé Mortet (PhD 2025)




















