Research interests
Our team investigates the pathophysiological mechanisms of Malformations of Cortical Development (MCDs). MCDs correspond to either macroscopic (e.g. subcortical band heterotopia) or microscopic abnormalities of the cerebral cortex that arise as a consequence of an interruption to the normal steps of formation of the cortical network. Indeed, normal cortical development requires a very precise and orchestrated mobilisation of various neuron subtypes arising from different regions of the brain, and born at various times, in order to achieve specific laminar positioning and connections with other cells. Until the availability of magnetic resonance imaging, MCDs were thought to be extremely rare disorders. It is now recognized that MCDs are a significant cause of epilepsy (20-40% of drug-resistant childhood epilepsies) and intellectual disability (ID), with most patients presenting in childhood. MCDs may be a result of genetic mutations and/or environmental causes such as in utero infection or ischemia. Mutations of many genes linked to several pathways known to regulate neuronal migration and maturation have been recently described in MCD patients, but the physiopathological mechanisms are poorly understood. Our goal is not only to increase our knowledge on genetically determined MCDs, but also to develop more effective therapeutic strategies for treating seizures and other MCDs based on the comprehension of the pathophysiological mechanisms. For this purpose, we conduct integrated multidisciplinary studies involving morphologists, molecular biologists and electrophysiologists. We have also established national and international collaborations with clinicians and geneticists (through the European consortium “DESIRE”, the EPINEXT”–DHU Aix-Marseille University network and the “DECIPHER”-EraNet Neuron funded project) providing us with a transversal appreciation of our researches.
Specific projects
- aMolecular mechanisms that underlie functional cortical network development in health and disease.
- bNeuronal basis of epileptic seizure generation and new therapies.
- cDevelopmental epilepsies and encephalopathies of genetic and viral origins
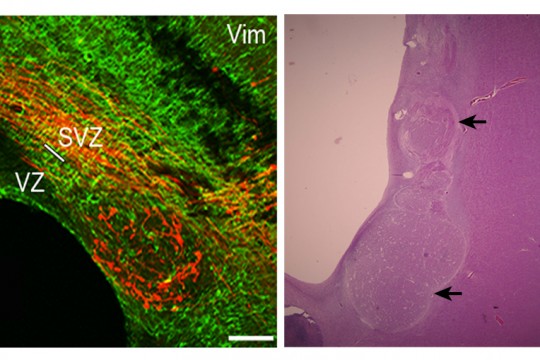
Molecular mechanisms that underlie functional cortical network development in health and disease.
Our aim is to decipher the mechanisms that control cortical network development through investigation of genetic mutations associated with MCDs. More precisely, we propose to investigate if and how mutations in MCD genes are involved in the morpho-functional changes underlying the development of cortical connections and networks by answering the following questions: i) what are the roles of MCD genes in neuronal migration?; ii) are these genes involved in axon outgrowth, dendritogenesis or synaptogenesis ?; iii) what are the consequences of mutations in MCD genes on morphology and functional maturation of cortical neurons ?
Neuronal basis of epileptic seizure generation and new therapies.
Our group has accumulated several evidence in favor of a common underlying mechanism for altered excitability in MCD rodent models, namely an altered neocortical excitation-inhibition (E/I) balance. Our data also indicate that cortical areas surrounding the heterotopia undergo reactive-type changes that severely modify E/I balance and affect neocortical circuitry, thus contributing to epileptogenesis. Our objectives are to precisely identify the seizure-generating zone, to describe its properties and the mechanisms of seizure generation, and to suggest new therapeutic approaches.
Developmental epilepsies and encephalopathies of genetic and viral origins
P Szepetowski (PI) and S Bauer (PI)
With N Burnashev, S Tarhini, E Buhler, PBMC
We aim at studying and at targeting the pathological alterations of genetic or nongenetic origin (e.g. viral infections) that occur early in the developing brain and that may have severe and long-term consequences on brain development and functioning.
Key words
Brain development, Epilepsy, Cerebral Cortex, Malformation of Cortical Development, Neuronal Migration, Animal ModelsExperimental approaches
Histological and Neuroanatomical Methods, Cellular and Molecular Biology (RNAi interference, in utero electroporation), Cell and Slice Culture, Extracellular Multi-site Recordings with Microelectrode Arrays, Time-lapse ImagingCollaborators and past memberss
Collaborators: – Renzo Guerrini (Florence University, Italy), – Massimo Pasqualetti (Pisa University, Italy) – Heiko Luhman (Mainz University, Germany) – Jamel Chelly (Institut Cochin, Paris, France) – William B. Dobyns (University of Chicago, USA) – Rikke S. Moller (University of Copenhagen, Denmark) – Hiroshi Kawasaki (University of Tokyo, Japan) Past members: – James Ackman (Yale University) – Sabrina Khantane (Invitrogen) – Irina Popova – Aurélie Carabalona (Columbia University)Funding
– Communauté Européenne (EC contract number LSH-CT-2006-037315 (EPICURE) FP6 – Thematic priority LIFESCIHEALTH) – ANR: 2006 neurosciences neurologie et phychiatrie (ANR-06-NEURO-008-01 contract number RPV06055ASA) – ANR: 2009 Programme Retour Post-doctorants (contract number RPV09010AAA) to JB Manent – Fondation J Lejeune to JB Manent – FRC Rotary to A Represa – Europe/INSERM/Région PACA – PhD fellowship to A CarabalonaJoin our team
Our team has open positions for undergraduate students, PhD students and postdoctoral fellows. Applications should be sent to Carlos Cardoso or Alfonso Represa.Alumni
Catia Palminha, thèse 2019 Antonin Vinck, ingénieur 2020
Delphie Hardy, Post-doct 2022 Rémi Mathieu, thèse 2022
Karen Runge, thèse 2022 J-C Vermoyal, thèse 2023
Nagham Badreddine, postdoc 2023 Léa Corbières, thèse 2024















