Therapeutic potential of cannabidiol polypharmacology in neuropsychiatric disorders

Trends in Pharmacological Sciences
Cannabidiol (CBD), the primary non-intoxicating compound in cannabis, is currently approved for treating rare, treatment-resistant seizures. Recent preclinical research suggests that CBD’s multifaceted mechanisms of action in the brain, which involve multiple molecular targets, underlie its neuroprotective, anti-in ammatory, anxiolytic, and antipsychotic effects. Clinical trials are also exploring CBD’s therapeutic potential beyond its current uses.
This review focuses on CBD’s polypharmacological profile and discusses the latest preclinical and clinical findings regarding its efficacy in neuropsychiatric disorders. Existing evidence suggests that CBD’s ability to modulate multiple signaling pathways may benefit neuropsychiatric disorders, and we propose further research areas to clarify its
mechanisms, address data gaps, and refine its therapeutic indications.
Single use of synthetic cannabinoids has lasting impact on the adolescent brain

Biological Psychiatry / Reelin Deficiency and Synaptic Impairment in Adolescent PFC following Initial Synthetic Cannabinoid Exposure
Even a single use of drugs like cannabis or synthetic cannabinoids can have a lasting impact on the brain. This is especially true for adolescents, whose brains are still developing.
The prefrontal cortex, the part of the brain responsible for decision-making, impulse control, and social behavior, is particularly vulnerable during this period. Exposure to synthetic cannabinoids can disrupt its normal development, leading to long-term cognitive and behavioral problems.
A key protein involved in brain development and function, Reelin, is affected by synthetic cannabinoid use. When Reelin levels are reduced, it can lead to problems with synaptic communication, balance between excitatory and inhibitory signals, and brain plasticity. These disruptions can contribute to the behavioral issues seen in people who use synthetic cannabinoids.
To understand the impact of synthetic cannabinoids on the brain, researchers studied adolescent mice. They found that a single dose of a synthetic cannabinoid can significantly decrease Reelin levels in specific parts of the prefrontal cortex. This reduction wasn’t due to decreased production but rather increased breakdown of the protein.
Interestingly, cannabinoid receptors and Reelin are often found together in the same brain cells. The researchers confirmed that the reduction in Reelin caused by synthetic cannabinoids is mediated by these cannabinoid receptors.
The decrease in Reelin led to a reduction in long-term potentiation, a process crucial for learning and memory. This effect was similar to what is observed in mice with reduced Reelin levels. However, when Reelin was directly infused into the prefrontal cortex, long-term potentiation was restored, demonstrating a direct link between Reelin depletion and synaptic dysfunction.
In conclusion, this study highlights the significant impact of a single synthetic cannabinoid exposure on brain development, particularly the prefrontal cortex, and identifies Reelin as a key molecular target involved in these detrimental effects.
Gestational CBD Shapes Insular Cortex in Adulthood

Cells
Many expectant mothers use CBD to relieve symptoms such as nausea, insomnia, anxiety, and pain, despite limited research on its long-term effects. However, CBD crosses the placenta, which can affect fetal development and influence children’s behavior. We studied how prenatal exposure to CBD affects the insular cortex (IC), a brain region involved in emotional processing and linked to psychiatric disorders.
The IC is divided into two parts: the anterior insular cortex (aIC), which processes socio-emotional signals, and the posterior insular cortex (pIC), specialized in interoception and pain perception. Pyramidal neurons in the aIC and pIC exhibit electrophysiological characteristics that vary by sex, particularly regarding excitability and the balance between excitatory and inhibitory signals.
We examined the cellular properties of the IC and the strength of synaptic connections in offspring of both sexes from mice exposed to low doses of CBD during gestation (E5–E18; 3 mg/kg, s.c.). Prenatal exposure to CBD led to sex-specific and region-specific changes in membrane properties and excitability, as well as in the excitatory/inhibitory balance in adult offspring. The results indicate that in utero exposure to CBD disrupts neuronal development in the IC, resulting in a loss of functional distinction between different regions of the IC. These findings could have important implications for understanding how CBD affects emotional behaviors in children.
Adaptive Group Behavior of Fragile X Mice in Unfamiliar Environments

Gabriele Giua, Benjamin Strauss, Olivier Lassalle, Pascale Chavis, Olivier J. Manzoni
Fragile X Syndrome (FXS) stands out as a prominent cause of inherited intellectual disability and a prevalent disorder closely linked to autism. FXS is characterized by substantial alterations in social behavior, encompassing social withdrawal, avoidance of eye contact, heightened social anxiety, increased arousal levels, language def-
icits, and challenges in regulating emotions. Conventional behavioral assessments primarily focus on short-term interactions within controlled settings. In this study, we conducted a comprehensive examination of the adaptive
group behavior of Fmr1 KO male mice over a three-day period, without introducing experimental interventions or task-based evaluations. The data unveiled intricate behavioral anomalies, with the most significant changes manifesting during the initial adaptation to unfamiliar environments. Notably, certain behaviors exhibited a
gradual return to typical patterns over time. This dynamic Fmr1 KO phenotype exhibited heightened activity, featuring increased exploration, amplified social interest, and an unconventional approach to social interactions
characterized by a higher frequency of shorter engagements. These findings contribute to the growing understanding of social behavior in individuals with FXS and underscore the significance of comprehending their adaptive responses in various environmental contexts.
Our research in the News

Long-term effects of gestational Cannabidiol
https://www.fens.org/news-activities/news/fens-forum-media-programm
https://medicalxpress.com/news/2024-06-safety-cannabidiol-pregnant-women.html
Cell- and pathway-specific disruptions in the accumbens of Fragile X mouse

Gabriele Giua, Olivier Lassalle, Pascale Chavis, Olivier J. Manzoni
Fragile X syndrome (FXS) is a genetic cause of intellectual disability and autism spectrum disorder (ASD), associated with social deficits. The mesocorticolimbic system, which includes the prefrontal cortex (PFC), basolateral amygdala (BLA), and nucleus accumbens core (NAcC), is essential for regulating socio-emotional behaviors.
We employed optogenetics to compare the functional properties of the BLA→NAcC, PFC→NAcC, and reciprocal PFC↔BLA pathways in Fmr1−/y::Drd1a-tdTomato mice. In FXS mice, the PFC↔BLA reciprocal pathway was unaffected, while significant synaptic modifications occurred in the BLA/PFC→NAcC pathways. We observed distinct changes in D1 striatal projection neurons (SPNs) and separate modifications in D2 SPNs. In FXS mice, the BLA→NAcC-D2 SPNs pathway demonstrated heightened synaptic strength, attributed to augmented AMPAR and NMDAR currents, along with an elevation in spine density that was specifically observed in D2 SPNs. Conversely, the amplified firing probability of BLA→NAcC-D1 SPNs was not accompanied by increased synaptic strength, AMPAR and NMDAR currents, or spine density. These pathway-specific alterations resulted in an overall enhancement of excitatory-to-spike coupling, a physiologically relevant index of how efficiently excitatory inputs drive neuronal firing, in both BLA→NAcC-D1 and BLA→NAcC -D2 pathways. Finally, the absence of FMRP led to impaired long-term depression specifically in BLA→D1 SPNs. These distinct alterations in synaptic transmission and plasticity within circuits targeting the NAcC highlight the potential role of postsynaptic mechanisms in selected SPNs in the observed circuit-level changes. This research underscores the heightened vulnerability of the NAcC in the context of FMRP deficiency, emphasizing its pivotal role in the pathophysiology of FXS.

Sexual differences in neuronal and synaptic properties across subregions of the mouse insular cortex
Listen to the Science Cast here
Background: The insular cortex (IC) plays a pivotal role in processing interoceptive and emotional information, offering insights into sex differences in behavior and cognition. The IC comprises two distinct subregions: the anterior insular cortex (aIC), that processes emotional and social signals, and the posterior insular cortex (pIC), specialized in interoception and perception of pain. Pyramidal projection neurons within the IC integrate multimodal sensory inputs, influencing behavior and cognition. Despite previous research focusing on neuronal connectivity and transcriptomics, there has been a gap in understanding pyramidal neurons characteristics across subregions and between sexes.
Methods: Adult male and female C57Bl/6J mice were sacrificed and tissue containing the IC was collected for ex vivo slice electrophysiology recordings that examined baseline sex differences in synaptic plasticity and transmission within aIC and pIC subregions.
Results: Clear differences emerged between aIC and pIC neurons in both males and females: aIC neurons exhibited distinctive features such as larger size, increased hyperpolarization, and a higher rheobase compared to their pIC counterparts. Furthermore, we observed variations in neuronal excitability linked to sex, with male pIC neurons displaying a greater level of excitability than their female counterparts. We also identified region-specific differences in excitatory and inhibitory synaptic activity and the balance between excitation and inhibition in both male and female mice. Adult females demonstrated greater synaptic strength and maximum response in the aIC compared to the pIC. Lastly, synaptic long-term potentiation occurred in both subregions in males but was specific to the aIC in females.
Conclusions: We conclude that there are sex differences in synaptic plasticity and excitatory transmission in IC subregions, and that distinct properties of IC pyramidal neurons between sexes could contribute to differences in behavior and cognition between males and females.
Sex-specific modulation of early life vocalization and cognition by Fmr1 gene dosage in a mouse model of Fragile X Syndrome

Gabriele Giua, Daniela Iezzi, Alba Caceres-Rodriguez, Benjamin Strauss, Pascale Chavis, Olivier J. Manzoni
Background
Pup-dam ultrasonic vocalizations (USVs) are essential to cognitive and socio-emotional development. In autism and Fragile X Syndrome (FXS), disruptions in pup-dam USV communication hint at a possible connection between abnormal early developmental USV communication and the later emergence of communication and social deficits.
Methods
Here, we gathered USVs from PND 10 FXS pups during a short period of separation from their mothers, encompassing animals of all possible genotypes and both sexes (i.e., Fmr1-/y vs. Fmr1+/y males and Fmr1+/+, +/-, and -/- females). This allowed comparing the influence of sex and gene dosage on pups’ communication capabilities. Leveraging DeepSqueak and analyzing vocal patterns, intricate vocal behaviors such as call structure, duration, frequency modulation, and temporal patterns were examined. Furthermore, homing behavior was assessed as a sensitive indicator of early cognitive development and social discrimination. This behavior relies on the use of olfactory and thermal cues to navigate and search for the maternal or nest odor in the surrounding space.
Results
The results show that FMRP-deficient pups of both sexes display an increased inclination to vocalize when separated from their mothers, and this behavior is accompanied by significant sex-specific changes in the main features of their USVs as well as in body weight. Analysis of the vocal repertoire and syntactic usage revealed that Fmr1 gene silencing primarily alters the USVs’ qualitative composition in males. Moreover, sex-specific effects of Fmr1 silencing on locomotor activity and homing behavior were observed. FMRP deficiency in females increased activity, reduced nest-reaching time, and extended nest time. In males, it prolonged nest-reaching time and reduced nest time without affecting locomotion.
Conclusions
These findings highlight the interplay between Fmr1 gene dosage and sex in influencing communicative and cognitive skills during infancy.

Cocaine-induced loss of LTD and social impairments are restored by fatty acid amide hydrolase inhibition
listen to the Science Cast here!
Background A single usage of a drug of abuse can have lasting effects on both the brain and behavior, continuing even after the drug has been metabolized and eliminated from the body. A single dose of cocaine can abolish endocannabinoid-mediated long-term depression (eCB-LTD) in the nucleus accumbens (NAc) within 24 hours of administration. However, it is uncertain whether this altered neuroplasticity entails a behavioral deficit.
Methods Our study employed adult male mice to investigate the effects of a single dose of cocaine (20 mg/kg) on eCB-LTD, saccharin preference, and social interactions 24 hours after administration. We also examined the gene expression in components of the eCB system. The pharmacological increase of anandamide was evaluated using the fatty acid amide hydrolase (FAAH) inhibitor URB597 (1 mg/kg).
Results After a single dose of cocaine, mice displayed altered plasticity, social interactions, and preference for saccharin and a reduction in mRNA levels of the anandamide-catabolizing enzyme NAPE-PLD. We discovered that the FAAH inhibitor URB597 (1 mg/kg) successfully reversed the cocaine-induced loss of eCB-LTD in the NAc and restored normal social interaction in cocaine-exposed mice, but it did not affect their saccharin preference.
Conclusions Overall, this research underlines the neuroplastic changes and subsequent behavioral alterations that occur after the initial use of cocaine, while also suggesting a potential role for anandamide in the early impairments caused by cocaine. The findings highlight the importance of understanding the mechanisms underlying the initiation of drug use and offer a potential therapeutic target.

Custom made python LMT scripts Chavis-Manzoni team
https://gitlab.com/chavis_manzoni_lab/lmt-scripts
all scripts by Benjamin Strauss
Cell-specific effects of FMRP deficiency on spiny neurons of the accumbens
G. Giua, O. Lassalle, L. Makrini-Maleville, E. Valjent, P. Chavis and O. Manzoni
Fragile X syndrome (FXS) is a major genetic cause of autism and intellectual disability due to a mutation in the Fmr1 gene. Patients with FXS have cognitive, emotional and social impairments, often associated with dysfunction of the nucleus accumbens (NAc), a brain region that regulates social behavior. The major cell type in the NAc, spiny neurons (SPNs), exist in two subtypes, differentiated by the expression of dopamine D1 or D2 receptors, their connectivity, and associated behavior.
We generated a new mouse model (Fmr1y/0: Drd1a-tdTomato) to examine how the absence of FMRP affects these SPN subtypes. We found Fmr1 and FMRP transcripts in both SPN subtypes, suggesting that Fmr1 functions may vary between cells. In mice with the Fmr1 mutation, membrane properties and action potential kinetics that normally differ between D1 and D2 SPNs were either reversed or completely abolished. The analysis revealed that the Fmr1 mutation significantly alters the phenotypic traits that distinguish each cell type in healthy mice.
Thus, the absence of FMRP in FXS mice disrupts the differences between NAc D1 and D2 SPNs, leading to a uniform cell phenotype. This uniformity may contribute to some aspects of this pathology.
Sex-specific maturational trajectory of endocannabinoid plasticity in the rat prefrontal cortex

Axel Bernabeu, Anissa Bara, Antonia Manduca, Milene Borsoi, Olivier Lassalle, Anne-Laure Pelissier-Alicot and OJJ Manzoni
The present study sheds light on the developmental differences in prefrontal cortex synaptic development between male and female rats. Our findings indicate that there are sex-specific differences in the excitability of PFC layer 5 pyramidal neurons, with adult females exhibiting lower excitability than other developmental stages. Additionally, we observed sexually dimorphic developmental patterns in endocannabinoid-mediated long-term depression, suggesting that sex hormones play a role in synaptic plasticity during adolescence.
The engagement of endovanilloid TRPV1R or eCB receptors during LTD was sequential and sexually dimorphic, indicating that the mechanisms underlying LTD are different between male and female rats. Moreover, the gene expression of the eCB/vanilloid systems was also sequential and sex-specific, with females exhibiting a different pattern of gene expression compared to males. These findings highlight the importance of considering sex differences in prefrontal cortex synaptic development and the need for further research in this area.
Our study also identified specific molecular targets that could be used to enhance LTD in young males, which could have implications for the treatment of neuropsychiatric disorders that are associated with deficits in prefrontal cortex synaptic plasticity. Specifically, our results suggest that inhibition of ABHD6 or MAGL could enhance LTD in young males. These findings could have important implications for developing new pharmacological treatments for conditions such as schizophrenia, bipolar disorder, and depression, which have been associated with altered prefrontal cortex synaptic plasticity.
In summary, our study provides new insights into the mechanisms underlying prefrontal cortex synaptic development and highlights the importance of considering sex differences in this process. Our findings also identify potential molecular targets for enhancing LTD in young males and provide a basis for future research in this area.
In utero exposure to cannabidiol disrupts select early-life behaviors in a sex-specific manner

Iezzi, Caceres-Rodriguez, Chavis & Manzoni
Cannabidiol (CBD), one of the main components of cannabis, is generally considered safe. CBD crosses the placenta and its use during pregnancy is steadily increasing, the impact of gestational CBD’s effects on prenatal life and neurodevelopment are poorly understood. Here, we combined behavioral approaches and deep learning analysis to assess the sex-dependent neonatal behavior of CBD exposed progeny. Gestating C57BL6/J dams were exposed daily with vehicle or CBD (3 mg/Kg, s.c.), from gestational day 5 to 18. Body weight, pup ultrasound vocalizations (USVs, PND 10) and homing behavior (PND 13) were quantified in the progeny. Thus, male (but not female) pups from CBD-treated dams gained more weight than sham. There were sex-dependent differences in the coarse characteristics of ultrasonic vocalizations. Prenatally-CBD exposed male pups emitted shorter calls, whereas CBD females made more high frequency calls when compared with their control counterparts. There were significant qualitative changes in the syllabic USV repertoire reflected in call typologies and communication patterns. Finally, the homing behavior test showed that CBD- exposed females presented a greater vulnerability to gestational CBD than males. Only CBD-exposed female pups showed reduced motor and discriminatory abilities. Together the results suggest a sexual divergence in the consequences of in utero CBD exposure on neonates at early developmental ages, which may be predictive of adult psychopathology. Given the extent of cannabis and CBD use worldwide, these findings challenge the idea that CBD is a universally safe compound and reveal the need for additional
studies on the effect of perinatal CBD exposure.
Translational Psychiatry (2022)12:501 ; https://doi.org/10.1038/s41398-022-02271-8
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) the NIH (R01DA043982 to O.M.), IReSP and INCa in the framework of a call for doctoral grant applications launched in 2022 (SPADOC22-003).
Cannabis in Adolescence: Lasting Cognitive Alterations and Underlying Mechanisms

Cannabis & Cannabinoid Research (2022)
Cannabis consumption during adolescence is an area of particular concern, owing to changes in the social and political perception of the drug, and presents a scientific, medical, and economic challenge. Major social and economic interests continue to push toward cannabis legalization as well as pharmaceutical development. As a result, shifting perceptions of both legal and illicit cannabis use across the population have changed the collective evaluation of the potential dangers of the product. The wave of cannabis legalization therefore comes with new responsibility to educate the public on potential risks and known dangers associated with both recreational and medical cannabis. Among these is the risk of long-term cognitive and psychological consequences, particularly following early-life initiation of use, compounded by high-potency and/or synthetic cannabis, and heavy/frequent use of the drug. Underlying these cognitive and psychiatric consequences are lasting aberrations in the development of synaptic function, often secondary to epigenetic changes. Additional factors such as genetic risk and environmental influences or nondrug toxic insults during development are also profound contributors to these long-term functional alterations following adolescent cannabis use. Preclinical studies indicate that exposure to cannabinoids during specific windows of vulnerability (e.g., adolescence) impacts neurodevelopmental processes and behavior by durably changing dendritic structure and synaptic functions, including those normally mediated by endogenous cannabinoids and neuronal circuits.
Cannabis and the Developping Brain 1st Edition

New book, now available
Cannabis and the Developing Brain provides comprehensive research on the effects of cannabis during neurodevelopment stages (i.e., perinatal and adolescent ages). The book introduces readers to vivo neural circuits and molecular and cellular mechanisms affected by cannabis exposure during three different temporal windows of brain vulnerability. In addition, it offers unique insights on shared neurobiological features of cannabinoid exposure during different developmental periods. Lastly, the book determines the adverse impact of developmental cannabinoid exposure on specific cognition, emotion and behaviors. Marijuana is the most commonly used psychotropic drug in the United States after alcohol. According to a 2018 NIH report, more than 11.8 million young adults reported marijuana use. With the legalization and decriminalization of cannabis, momentum continues to build and be propelled by the reduction of stigma associated to its consumption, hence there is growing concern regarding long-term impacts on brain function and behavior.
Characterization of Nasco grape pomace-loaded nutriosomes and their neuroprotective effects in the MPTP mouse model of Parkinson’s disease

Parekh, Serra, Allaw, Perra, Marongiu, Tolle, Pinna, Casu, Manconi, Caboni, Manzoni & Morelli
Grape pomaces have recently received great attention for their richness in polyphenols, compounds known to exert anti-inflammatory and antioxidant effects. These pomaces, however, have low brain bioavailability when administered orally due to their extensive degradation in the gastrointestinal tract. To overcome this problem, Nasco pomace extract was incorporated into a novel nanovesicle system called nutriosomes, composed of phospholipids (S75) and water-soluble maltodextrin (Nutriose® FM06). Nutriosomes were small, homogeneously dispersed, had negative zeta potential, and were biocompatible with intestinal epithelial cells (Caco-2). Nasco pomace extract resulted rich in antioxidant polyphenols (gallic acid, catechin, epicatechin, procyanidin B2, and quercetin). To investigate the neuroprotective effect of Nasco pomace in the subacute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease (PD), Nasco nutriosomes or Nasco suspension was administered intragastrically and their neuroprotective effects were evaluated. Degeneration of nigro-striatal dopaminergic neurons induced by subacute MPTP treatment, the pathological hallmark of PD, was assessed through immunohistochemical evaluation of tyrosine hydroxylase (TH) in the caudate-putamen (CPu) and substantia nigra pars compacta (SNc), and the dopamine transporter (DAT) in CPu. Immunohistochemical analysis revealed that Nasco nutriosomes significantly prevented the reduction in TH- and DAT- positive fibres in CPu, and the number of TH-positive cells in SNc following subacute MPTP treatment, while Nasco suspension counteracted MPTP toxicity exclusively in SNc. Overall, these results highlight the therapeutic effects of Nasco pomace extract when administered in a nutriosome formulation in the subacute MPTP mouse model of PD and validate the effectiveness of the nutriosome preparation over suspension as an innovative nano-drug delivery system for in vivo administration
Cannabidiol and substance use disorder: Dream or reality
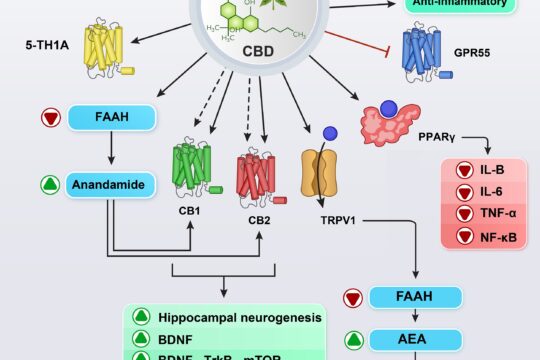
Karimi-Haghighiab, Razavi, Iezzi, Scheyer, Manzoni, Haghparast
Cannabidiol (CBD) is one of the major constituents of Cannabis sativa L. that lacks psychotomimetic and rewarding properties and inhibits the rewarding and reinforcing effects of addictive drugs such as cocaine, methamphetamine (METH), and morphine. Additionally, CBD’s safety profile and therapeutic potential are currently evaluated in several medical conditions, including pain, depression, movement disorders, epilepsy, multiple sclerosis, Alzheimer’s disease, ischemia, and substance use disorder. There is no effective treatment for substance use disorders such as addiction, and this review aims to describe preclinical and clinical investigations into the effects of CBD in various models of opioid, psychostimulant, cannabis, alcohol, and nicotine abuse. Furthermore, the possible mechanisms underlying the therapeutic potential of CBD on drug abuse disorders are reviewed.
Sex-specific divergent maturational trajectories in the postnatal rat basolateral amygdala
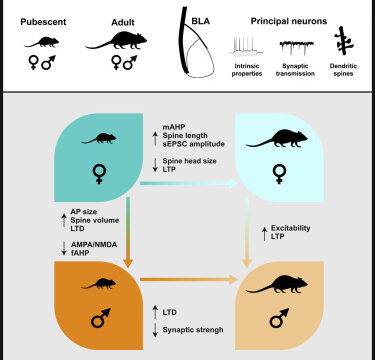
Guily, Lassalle, Chavis & Manzoni
The basolateral amygdala (BLA), the part of the amygdala complex involved in the transduction of perceptual stimuli into emotion, undergoes profound reorganization at adolescence in rodents and humans. How cellular and synaptic plasticity evolve throughout postnatal development in both sexes is only partially understood. We used a cross-sectional approach to compare the morphology, neuronal, and synaptic properties of BLA neurons in rats of both sexes at adolescence and adulthood. While BLA pyramidal neurons from rats of both sexes displayed similar current-voltage relationships, rheobases, and resting potentials during pubescence, differences in these parameters emerged between sexes at adulthood: BLA neurons were more excitable in males than females. During pubescence, BLA neuron excitability was highest in females and unchanged in males; male action potentials were smaller and shorter than females and fast afterhyperpolarizations were larger in males. During post-natal maturation, no difference in spine density was observed between groups or sexes but spine length increased and decreased in females and males, respectively. A reduction in spine head diameter and volume was observed exclusively in females. Basic synaptic properties also displayed sex-specific maturational differences. Stimulus-response relationships and maximal fEPSP amplitudes where higher in male adolescents compared with adults but were similar in females of both ages. Spontaneous excitatory postsynaptic currents mediated by AMPA receptors were smaller in BLA neurons from adolescent female compared with their adult counterparts but were unchanged in males. These differences did not directly convert into changes in overall synaptic strength estimated from the AMPA/NMDA ratio, which was smaller in adolescent females. Finally, the developmental courses of long-term potentiation and depression (LTP, LTD) were sexually dimorphic. LTP was similarly present during the adolescent period in males and females but was not apparent at adulthood in females. In contrast, LTD followed an opposite development: present in adolescent females and expressed in both sexes at adulthood. These data reveal divergent maturational trajectories in the BLA of male and female rats and suggest cellular substrates to the BLA linked sex-specific behaviors at adolescence and adulthood.
Perinatal THC Exposure via Lactation Induces Lasting Alterations to Social Behavior and Prefrontal Cortex Function in Rats at Adulthood

Perinatal THC Exposure via Lactation Induces Lasting Alterations to Social Behavior and Prefrontal Cortex Function in Rats at Adulthood
Cannabis is the world’s most widely abused illicit drug and consumption amongst women during and surrounding the period of pregnancy is increasing. Previously, we have shown that cannabinoid exposure via lactation during the early postnatal period disrupts early developmental trajectories of prefrontal cortex maturation and induces behavioral abnormalities during the first weeks of life in male and female rat progeny. Here, we investigated the lasting consequences of this postnatal cannabinoid exposure on synaptic and behavioral parameters in the adult offspring of ∆9-tetrahydrocannabinol (THC)-treated dams. At adulthood, these perinatally THC-exposed rats exhibits deficits in social discrimination accompanied by an overall augmentation of social exploratory behavior. These behavioral alterations were further correlated with multiple abnormalities in synaptic plasticity in the prefrontal cortex, including lost endocannabinoid-mediated long-term depression (LTD), lost long-term potentiation and augmented mGlu2/3-LTD. Finally, basic parameters of intrinsic excitability at prefrontal cortex pyramidal neurons were similarly altered by the perinatal THC exposure. Thus, perinatal THC exposure via lactation induces lasting deficits in behavior and synaptic function which persist into adulthood life in male and female progeny.
Endocannabinoid LTD in accumbal D1 neurons mediates reward seeking behavior
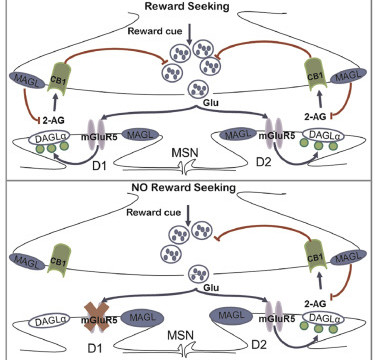
iScience https://doi.org/10.1016/j.isci.2020.100951
The nucleus accumbens (NAc) plays a key role in drug-related behavior and natural reward learning. Synaptic plasticity in dopamine D1 and D2 receptors medium spiny neurons (MSNs) of the NAc and the endogenous cannabinoid (eCB) system have been implicated in reward-seeking. However, the precise molecular and physiological basis of reward-seeking behavior remains unknown. We found that the specific deletion of metabotropic glutamate receptor 5 (mGluR5) in D1-expressing MSN neurons (D1miRmGluR5 mice) abolishes eCB-mediated long-term depression (LTD) and prevents the expression of drug (cocaine and ethanol), natural reward (saccharin), and brain stimulation-seeking behavior. In vivo enhancement of 2-arachidonoylglycerol (2-AG) eCB signaling within the NAc core restores both eCB-LTD and reward-seeking behavior in D1miRmGluR5 mice. The data suggest a model where the eCB and glutamatergic systems of the NAc act in concert to mediate reward-seeking responses.
Cell-type and endocannabinoid specific synapse connectivity in the adult nucleus accumbens core
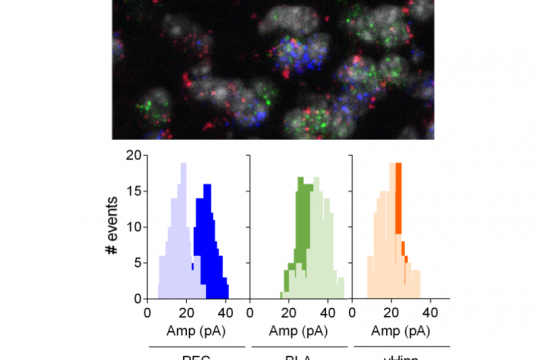
The Journal of Neuroscience 2019
The nucleus accumbens (NAc) is a mesocorticolimbic structure that integrates cognitive, emotional and motor functions. Although its role in psychiatric disorders is widely acknowledged, the understanding of its circuitry is not complete. Here we combined optogenetic and whole-cell recordings to draw a functional portrait of excitatory disambiguated synapses onto D1 and D2 medium spiny neurons (MSNs) in the adult male mouse NAc core. Comparing synaptic properties of ventral hippocampus (vHipp), basolateral amygdala (BLA) and prefrontal cortex (PFC) inputs revealed a hierarchy of synaptic inputs that depends on the identity of the postsynaptic target MSN. Thus, the BLA is the dominant excitatory pathway onto D1 MSNs (BLA > PFC = vHipp) while PFC inputs dominate D2 MSNs (PFC > vHipp > BLA). We also tested the hypothesis that endocannabinoids endow excitatory circuits with pathway- and cell-specific plasticity. Thus, while CB1 receptors (CB1R) uniformly depress excitatory pathways irrespective of MSNs identity, TRPV1 receptors (TRPV1R) bidirectionally control inputs onto the NAc core in a pathway-specific manner. Finally, we show that the interplay of TRPV1R/CB1R shapes plasticity at BLA-NAc synapses. Together these data shed new light on synapse and circuit specificity in the adult NAc core and illustrate how endocannabinoids contribute to pathway-specific synaptic plasticity.
A new international EU-funded project for our team

New Business opportunities & Environmental suSTainability using MED GRAPE nanotechnological products
Grape as a traditional crop in the Mediterranean area has a strong innovation potential, which has not been effectively exploited yet. People working in the grape cultivation sector are generally focused on improving the quality of grape and wine, but usually don’t consider how to make the most out of grape waste. Based on the R&D experience of the partners in the fields of grape valorization, waste exploitation and development of nanotechnological antioxidant/anti-inflammatory/anti-neurodegenerative formulations, BESTMEDGRAPE aims at supporting the creation of new startups/SMEs by transferring scientific/technological knowledge on local grape cultivars and the exploitation of wine by-products as a source of bioactive compounds that can be transformed into innovative commercial health products. Hence, the project will not only valorise a Mediterranean product – grape – but also the expansion of the grape value chain through the development of nanotechnological products, thus boosting the local economy, reducing environmental pollution and increasing employment opportunities. http://www.enicbcmed.eu/fr/node/525
Consequences of Perinatal Cannabis Exposure

Trends Neurosci. 2019
Series: Early-Life Stress and the Brain Review Get this article here
Cannabis exposure during the perinatal period results in varied and significant consequences in affected offspring. The prevalence of detrimental outcomes of perinatal cannabis exposure is likely to increase in tandem with the broadening of legalization and acceptance of the drug. As such, it is crucial to highlight the immediate and protracted consequences of cannabis exposure on pre- and postnatal development. Here, we identify lasting changes in neurons’ learning flexibility (synaptic plasticity) and epigenetic misregulation in animal models of perinatal cannabinoid exposure (using synthetic cannabinoids or active components of the cannabis plant), in addition to significant alterations in social behavior and executive functions. These findings are supported by epidemiological data indicating similar behavioral outcomes throughout life in human offspring exposed to cannabis during pregnancy. Further, we indicate important lingering questions regarding accurate modeling of perinatal cannabis exposure as well as the need for sex- and age-dependent outcome measures in future studies.
Cannabinoid exposure via lactation in rats disrupts perinatal programming of the GABA trajectory and select early-life behaviors

Biological Psychiatry
Cannabis usage is increasing with its widespread legalization. Cannabis use by mothers during lactation transfers active cannabinoids to the developing offspring during this critical period and alters postnatal neurodevelopment. A key neurodevelopmental landmark is the excitatory to inhibitory GABA switch caused by reciprocal changes in expression ratios of the K+/Cl- transporters KCC2 and NKCC1.
Treating rat dams with cannabinoids during early lactation retards transcriptional upregulation and expression of KCC2, thereby delaying the GABA switch in pups of both sexes. This perturbed trajectory was corrected by the NKCC1 antagonist bumetanide and accompanied by alterations in ultrasonic vocalization without changes in homing behavior. Neurobehavioral deficits were prevented by CB1R antagonism during maternal exposure, showing that CB1R underlie the cannabinoid-induced alterations.
These results reveal how perinatal cannabinoid exposure retards an early milestone of development, delaying the trajectory of GABA’s polarity transition and altering early-life communication.

Research Project
Our general aim is to understand how meso-corticolimbic (MCL) microcircuits are shaped throughout early life critical periods especially adolescence, to give rise to harmonious emotional behaviors and cognitive functions in adulthood. Specifically, we want to understand how environmental and genetic insults modeling neuropsychiatric diseases transform the architecture and the functionality of synaptic networks and reduce the behavioral working range.
Our previous work fueled the concept that structural and functional damages during early life periods including adolescence are causal in disease-linked behavioral deficits. Our core hypothesis is that adolescence delineates a period of maximal vulnerability and consequently is a critical determinant of how environments and genes shape neuronal network functions into adulthood (Bara et al. 2018; Labouesse et. al. 2017; Manduca et al. 2017; Bouamrane et al. 2017; Iafrati et al. 2016; Iafrati et al. 2014).
Our research project will allow disambiguating complex phenotypes into new developmental endophenotypes and the design of innovative therapeutic strategies.
Our project is organized in three objectives:
First, we systematically audit structural and functional properties to determine how development shapes MCL microcircuits.
Second, we use a strategy that we recently conceived, based on multivariate analysis of bootstrapped datasets (Iafrati et al. 2016) to consider the multidimensional nature of the data and evaluate the interrelationship between structural, functional and behavioral parameters.
Third, we use optogenetic stimulation and pharmacological modulation of specific neuronal microcircuits to recreate/compensate/reactivate adapted behavioral in diseased rodents.
Our multidisciplinary approach combines, electrophysiology, ablation by toxin receptor cell targeting of selected neuronal population, in vitro and in vivo calcium imaging, quantitative tridimensional neuroanatomy, optogenetics and the analysis of naturalistic behaviors across the emotional and cognitive domains.
Key words
Current Fundings
– NIH (co-P.I. O. MANZONI & K. MACKIE) – FRC (De CHEVIGNY, P. CHAVIS) – FRM (P.I. O. MANZONI)Fundings
Current Fundings
– NIH (co-P.I. O. MANZONI & K. MACKIE)
– FRC (De CHEVIGNY, P. CHAVIS)
– FRM (P.I. O. MANZONI)












